SARS-CoV-2 Nucleoprotein ELISA Kit
Due to the possibility of mismatching between antigens from other origin and antibodies used in our kits (e.g., antibody targets conformational epitope rather than linear epitope), some native or recombinant proteins from other manufacturers may not be recognized by our products.
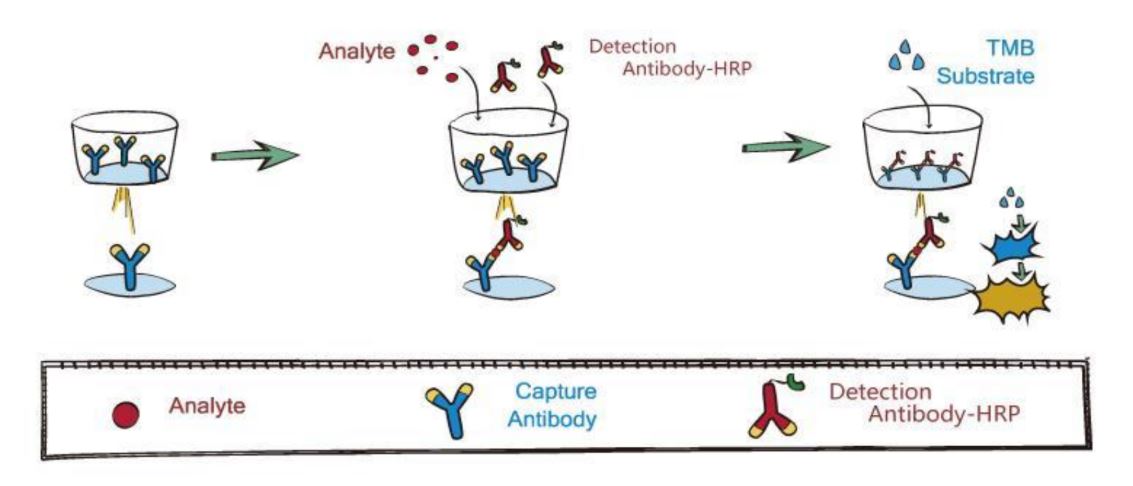
Principle of the Assay
This assay employs the quantitative sandwich enzyme immunoassay technique. A monoclonal antibody specific for SARS-CoV-2 Nucleoprotein has been pre-coated onto a microplate. Standards and samples are pipetted into the wells, and then a horseradish peroxidase-conjugated detection antibody specific for SARS-CoV-2 Nucleoprotein is added to the wells, producing an antibody-antigen-antibody "sandwich complex". Following incubation and wash steps a substrate is added. A colored product is formed in proportion to the amount of SARS-CoV-2 Nucleoprotein present in the sample. The reaction is terminated by the addition of acid and absorbance is measured at 450nm. A standard curve is prepared from six SARS-CoV-2 Nucleoprotein standard dilutions and SARS-CoV-2 Nucleoprotein sample concentration determined.
Target Information
Coronaviruses are enveloped viruses with a positive-sense RNA genome and with a nucleocapsid of helical symmetry. Coronavirus nucleoproteins localize to the cytoplasm and the nucleolus, a subnuclear structure, in both virus-infected primary cells and in cells transfected with plasmids that express N protein. Coronavirus N protein is required for coronavirus RNA synthesis and has RNA chaperone activity that may be involved in template switch. Nucleocapsid protein is the most abundant protein of coronavirus. During virion assembly, N protein binds to viral RNA and leads to the formation of the helical nucleocapsid. Nucleocapsid protein is a highly immunogenic phosphoprotein also implicated in viral genome replication and in modulating cell signaling pathways. Because of the conservation of the N protein sequence and its strong immunogenicity, the N protein of coronavirus is chosen as a diagnostic tool.
Materials Supplied
| Kit Components | 96 Wells Quantity/Size |
|---|---|
| Aluminum pouches with a Microwell Plate coated with monoclonal antibody to SARS-CoV-2 Nucleoprotein (8×12) |
1 plate |
| SARS-CoV-2 Nucleoprotein standard, 25ng/ml |
2 vials |
| Concentrated Detection antibody conjugated to horseradish peroxidase (HRP) |
2 vials |
| Standard /sample Diluent |
1 bottle |
| Detection antibody Diluent |
1 bottle |
| Wash Buffer Concentrate 20x (PBS with 1% Tween-20) | 1 bottle |
| Substrate Solution | 1 bottle |
| Stop Solution | 1 bottle |
| Adhesive Films | 4 pieces |
| Product data sheet | 1 copy |
Storage
| Storage | Store at 2 - 8°C |
Performance Characteristics
| REPEATABILITY | The coefficient of variation of both intra-assay and inter-assay was less than 10%. |
| SENSITIVITY | The minimum detectable dose was 0.4ng/mL. |
| SPECIFICITY | This assay can recognize both recombinant and natural SARS-CoV-2 Nucleoprotein,but will not react with recombinant MERS-CoV Nucleoprotein. |
Data Analysis Assistance
We have partnered with MyAssays to offer you an easy to use and versatile tool to analyze the data you receive using our ELISA Kit. Click the link below to be directed to the data analysis tool provided by MyAssays specifically for bskv0001.
https://www.myassays.com/bioss-sars-cov-2-nucleoprotein-elisa-kit.assay